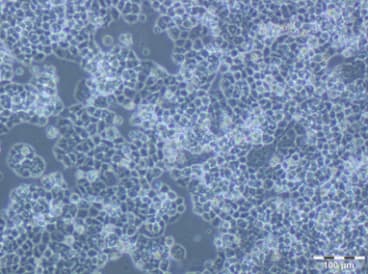
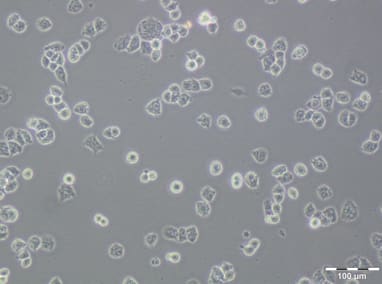
A2780 PTX(4) resistant cell line
Invented by Dr Jolanta Szenajch from Military Institute of Medicine, Warsaw, Poland , Dr Luiza Handschuh from Institute of Bioorganic Chemistry Polish Academy of Sciences , Dr Aleksandra Świercz from Institute of Bioorganic Chemistry Polish Academy of Sciences , Dr Michal Góralski from Institute of Bioorganic Chemistry Polish Academy of Sciences , Dr Alicja Szabelska-Beręsewicz from Poznań University of Life Science , Prof Idzi Siatkowski from Poznań University of Life Science , Dr Joanna Zyprych-Walczak from Poznań University of Life Science
Invented at Military Institute of Medicine, Warsaw, Poland
- Datasheet
- References (1)
- Inventor Info
Info
| Catalogue Number | 160729 |
| Antigen/Gene or Protein Targets | Paclitaxel resistance |
| Parental Line | A2780 |
| Host | Human |
| Tissue | Ovary |
| Disease Keywords | Ovarian Cancer |
| Model | Tumour line |
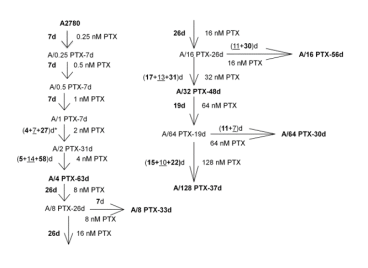
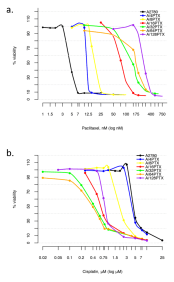
| Relevance | The paclitaxel (PTX) resistant A2780 PTX (4) cell line with collateral sensitivity to cisplatin (CDDP) (inverse resistance) has been developed by continuous growing of A2780 parental cell line (cat. no 152706) in step-wise increases of PTX doses (0.25 nM - 0.5 nM - 1 nM- 2 nM - 4 nM; for details see Fig. 1). The half maximal inhibitory concentration (IC50) for PTX is about twice as high the IC50 for A2780, whereas for CDDP it remains unchanged (for details see Tab. 1). PTX and CDDP are standard chemotherapeutic drugs used in the treatment of ovarian cancer and acquired resistance to them remains a major obstacle in therapy. The trend of developing the inverse resistance is dominant in clinical practice, therefore A2780 PTX (4) cell line with other sublines of the series (see Notes for details) can be used as a primary tool for deciphering the molecular and cellular mechanisms of drug resistance in ovarian cancer. |
| Production Details | Split sub-confluent cultures (70-80%) seeding at 4 x 1000 (tolerated range: 1 x 1000 to 1 x 10 0000) cells/cm2 preferably using cell dissociation solution (CDS: 0.3g Na2EDTA, 8 g NaCl, 0.56 g sodium bicarbonate, 1 g D-glucose and 0.4 g KCl per 1000 ml H2O; filter sterilized) or TrypLE ExpressTM, 5% CO2, 37oC. Culture cells without drug until they have been fully adhered (after 24 h). |
| Research Area | Cancer, Drug Discovery & Development |
| Growth/Phenotype Keywords | Adherent |
| Recommended Growing Conditions | RPMI 1640 medium + 2mM L-alanine-L-glutamine (GlutaMAXTM) + 5% heat-inactivated Foetal Bovine Serum (FBS) , at 37oC and 5% CO2 . To keep the stability of resistance profile not using antibiotics and antimycotics is recommended. Cells should be cultured without PTX (up to 10 days) to avoid the resistance escalation. After this time the maintenance treatment with 4 nM PTX for one passage is recommended. |
| Notes |
This cell line was generated as part of a series of isogenic ovarian cancer lines with gradually changing inverse resistance to PTX (resistant) and CDDP (sensitive); see A2780 PTX (8) Cat no:160730, A2780 PTX (16) Cat no:160731, A2780 PTX (32) Cat no:160732, A2780 PTX (64) Cat no:160733, and A2780 PTX (128) Cat no:160734 for the others (see Tab. 1 and Fig. 2 for details). Transcriptomes of six sublines were sequenced and the mRNA dataset was deposited under Gene Expression Omnibus Accession No GSE159791. Patent number: 233178 |
References: 1 entry
References: 1 entry
Inventor Information
Inventors

|
Dr Jolanta Szenajch |
|
from Military Institute of Medicine, Warsaw, Poland |

|
Dr Luiza Handschuh |
|
from Institute of Bioorganic Chemistry Polish Academy of Sciences |

|
Dr Aleksandra Świercz |
|
from Institute of Bioorganic Chemistry Polish Academy of Sciences |

|
Dr Michal Góralski |
|
from Institute of Bioorganic Chemistry Polish Academy of Sciences |