AzuFluorTM 483-Bpin: Azulene-Based Fluorescent Probe for ROS/RNS small molecule (tool compound)
Invented by Lloyd Murfin , Tony James , Simon Lewis
Invented at University Of Bath
- Datasheet
- References (1)
- Inventor Info
Info
| Catalogue Number | 157848 |
| Antigen/Gene or Protein Targets | Reactive Oxygen and Nitrogen Species |
| Synonyms | AzuFluorTM 483-Bpin, Diethyl 2-amino-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)azulene-1,3- dicarboxylate |
| Type | Fluorescent Probe |
| Relevance |
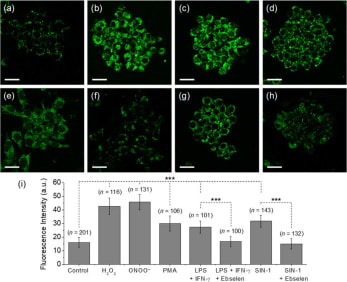
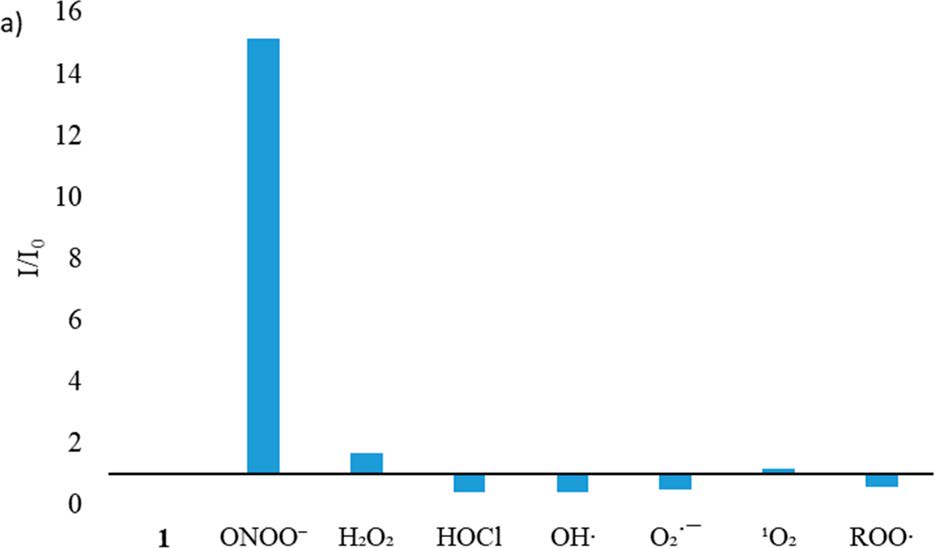
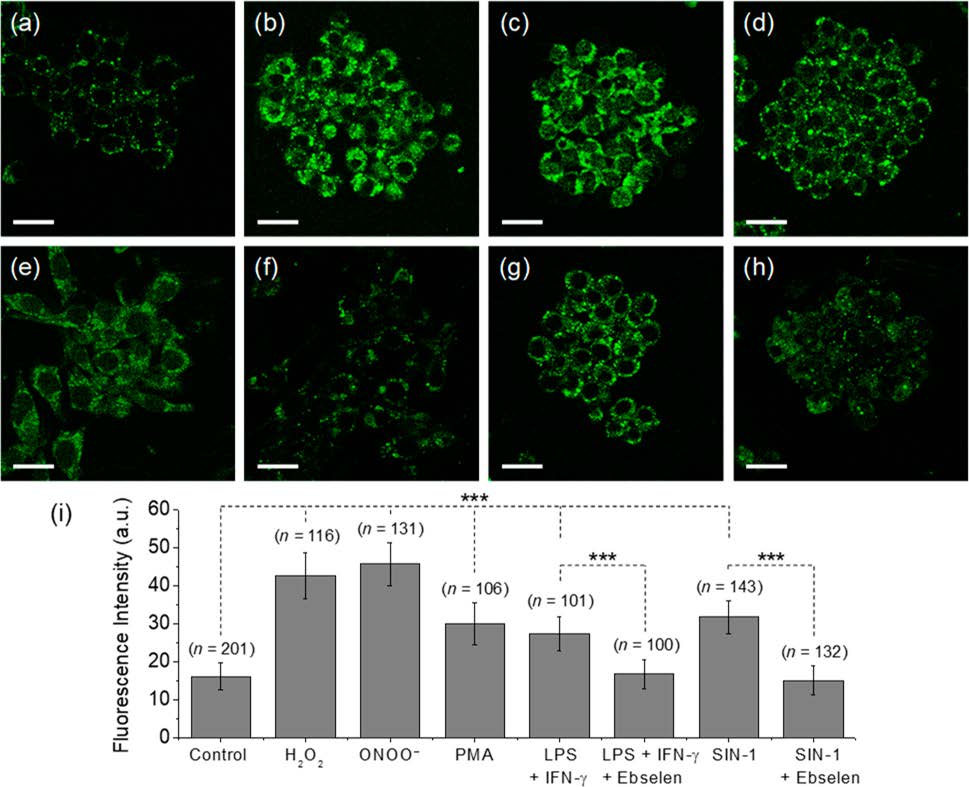
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are important mediators in many physiological and pathological processes. The one-electron reduction of O2 in vivo leads to the formation of O2•− (superoxide), which in turn undergoes disproportionation catalyzed by superoxide dismutase to give O2 and H2O2 (hydrogen peroxide). Another fate of O2•− is to react with endogenously produced NO (nitric oxide) to form ONOO− (peroxynitrite) via a nonenzymatic process. Ordinarily, the flux of H2O2 is tightly regulated; aberrant H2O2 production or overexposure is implicated in the pathogenesis of many diseases such as cancer and neurodegenerative conditions. Similarly, while ONOO− has roles in signal transduction, its strongly oxidizing and nitrating properties mean it can react in an uncontrolled manner with various biomolecules. Elevated levels of ONOO− have been linked to cardiovascular, neurodegenerative, and inflammatory diseases as well as cancer. In view of this, there is significant need for tools and techniques to elucidate the roles of ONOO−, H2O2, and other ROS/RNS in biological systems. |
| Molecular Formula | C22H28BNO6 |
| Key Attributes | AzuFluorTM 483B-pin shows high selectivity for peroxynitrite and hydrogen peroxide over other reactive oxygen species. It is cell-permeable, photostable, and noncytotoxic in vitro on the time scale of the two-photon fluorescence microscopy experiments. |
| Molecular Weight (g/mol) | 412.2046 |
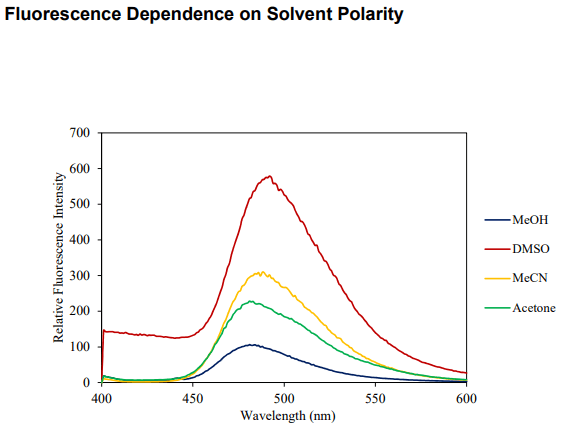
| Solubility | DMSO, MeCN, Acetone, MeOH |
| Research Area | Cancer, Cardiovascular, Neurobiology, Other |
| Storage | ambient |
| Notes |
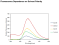
pH: Fluorescence intensity greatest at pH 7-8. Optimal two-photon excitation wavelength λex = 800nm. |
References: 1 entry
References: 1 entry
Inventor Information
Inventors

|
Lloyd Murfin |

|
Tony James |

|
Simon Lewis |