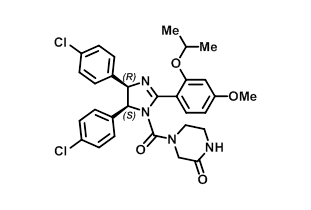
MDM2 inhibitor (+)-Nutlin 3b Small Molecule (Tool Compound)
Invented by Prof Bernard Golding from University of Newcastle upon Tyne
Invented at
- Datasheet
- References (4)
- Inventor Info
Info
| Antigen/Gene or Protein Targets | MDM2/p53 |
| Synonyms |
(+)-4-[4,5-Bis(4-chlorophenyl)-2-(2-isopropoxy-4-methoxyphenyl)-4,5-dihydro-1H-imidazole-1-carbonyl]piperazin-2-one CAS No. 675576-97-3 |
| Type | Inhibitor |
| Relevance | MDM2 inhibitor (+)-Nutlin 3b is useful as a negative control s for non-MDM2-related cellular activities.Loss of p53 activity, by deletion, mutation, or MDM2 overexpression, is the most common event in the development and progression of cancer, while the restoration of endogenous p53 function results in tumor regression in vivo. In this context, the rescue of the impaired p53 activity and resensitization to apoptosis in cancer cells by disrupting the MDM2−p53 interaction offers an opportunity for anticancer therapeutics. |
| On Target IC50 | IC50 value of 13.6 μM, 150-fold less potent (+)-enantiomer of Nutlin-3 as in comparison with opposite (-)-enantiomer Nutlin-3a. |
| Molecular Formula | C30H30Cl2N4O4 |
| Molecular Weight (g/mol) | 581.49 |
| In vitro applications | Nutlin-3b is useful as a negative control s for non-MDM2-related cellular activities. Nutlin-3a induces the expression of MDM2 and p21 (but not p53) only in cells with wild-type p53. Nutlin-3b has no effect regardless of the p53 status of the cells. Only the active enantiomer Nutlin-3a shows a potent antiproliferative activity and clear separation of potency between the cells harboring wild-type p53 and those harboring mutant p53. The potency of Nutlin-3b is much lower in the wild-type p53 cells and nearly identical to the potency of Nutlin-3a against the mutant p53 cells. After 48 hours of exposure to the Nutlin-3a, 45% of the cell population became TUNEL positive, but cells treated with the Nutlin-3b are indistinguishable from the untreated controls. |
| Research Area | Cancer, Cell Cycle, Epigenetics & Nuclear Signalling |
References: 4 entries
Bertamino et al. 2013. J Med Chem. 56(13):5407-21. PMID: 23802716.
Synthesis, in vitro, and in cell studies of a new series of [indoline-3,2'-thiazolidine]-based p53 modulators.
Europe PMC ID: 23802716
Vassilev et al. 2004. Science. 303(5659):844-8. PMID: 14704432.
In vivo activation of the p53 pathway by small-molecule antagonists of MDM2.
Europe PMC ID: 14704432
Add a reference
References: 4 entries
Bertamino et al. 2013. J Med Chem. 56(13):5407-21. PMID: 23802716.
Synthesis, in vitro, and in cell studies of a new series of [indoline-3,2'-thiazolidine]-based p53 modulators.
Vassilev et al. 2004. Science. 303(5659):844-8. PMID: 14704432.
In vivo activation of the p53 pathway by small-molecule antagonists of MDM2.
Add a reference