Throughout the 1970s and early 1980s, the techniques of molecular biology were developing rapidly. With the discovery of restriction and ligation enzymes, early PCR techniques and the introduction of methods to create monoclonal antibodies, the molecular toolkit was expanding apace. These techniques have continued to be refined, and now, with the development of recombinant antibodies, the power of recombinant DNA technology is being applied to these core tools in proteomics as well.
Unlike monoclonal antibodies, recombinant antibodies are generated in
vitro using synthetic genes.
There are four main types of recombinant antibodies:
Full antibody backbone
These are full length antibodies containing both Fab (antigen-binding fragment) and Fc (fragment crystallizable) regions.
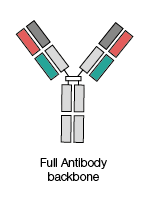
These consist of two sets of variable and constant components,which form together to create polypeptide chains.
Single chain variable fragment (scFv)
This is
the smallest type of recombinant antibody that is still capable of antigen
binding. It consists of a fusion protein of the variable regions of heavy (VH) and light chains (VL) of immunoglobulins. These
are then connected with a short linker peptide of about 10 to 25 amino acids.
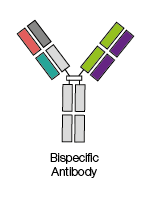
These antibodies combine two different antigen-binding sites
within one molecule, allowing these antibodies to be used by researchers to
target different antigens at the same time.
Knowing and understanding the differences between recombinant and monoclonal antibodies and the benefits and disadvantages of using each method is crucial. This will ensure you are selecting the best type of antibody to use within your research.
Producing antibodies
The biggest difference in producing de novo recombinant and monoclonal antibodies, is that monoclonal antibodies need to be produced using traditional hybridoma-based technologies while recombinant antibodies rely on phage display and recombinant DNA technology. This change in approach to antibody production will ultimately phase out the reliance on traditional animal and hybridoma methods. However, in addition to this, recombinant antibodies can be and are still currently generated through the sequencing of standard hybridomas produced in the traditional way. Once an antigen binding sequence has been identified and inserted into a vector, expression is accomplished through host expression systems, which can be a mammalian, bacterial or plant-based system. These different production methods provide some of the advantages of using recombinant antibodies vs. monoclonal antibodies.
Advantages of recombinant antibody production
There are several advantages of recombinant antibody production compared to monoclonal antibody production. These include:
Increased researcher control over the antigen specific sequence
Unlike monoclonal hybridoma antibody production, where control over an antibody’s antigen binding sequence is determined by the host animal to which the antigen is injected, recombinant antibodies have a defined sequence within a vector that can be easily manipulated by scientists. This means that researchers have greater control over the antigen binding sequence during recombinant antibody production, producing more reliable and reproducible antibodies in comparison to monoclonal antibodies.
Overcome the challenges of hybridoma stability
Converting existing hybridomas to recombinant antibody production allows the benefits of stability and reproducibility to be applied to the valuable catalogues of existing antibodies.
Quicker production time
This increase in a researcher’s control over the recombinant antibody production process also means that an antigen-specific antibody can be produced in a much shorter time frame, than can be achieved through monoclonal hybridoma technology. Recombinant antibodies can be produced in as short a timeframe as eight weeks This is unlike monoclonal hybridoma antibody production, which can require a minimum of four months. It is therefore a much shorter timescale from producing to using recombinant antibodies than it is for monoclonal antibodies.
Greater flexibility
It is very easy to convert the species, isotype or subtype of a recombinant antibody fragment simply by adding the appropriate constant domain.
Reduced ethical or animal welfare concerns
Animals don’t need to be used in the recombinant antibody production process when using the phage display method. This means that many ethical and animal welfare concerns, that are commonly associated with traditional monoclonal antibody products no longer apply.
There are also several applications where using recombinant antibodies can be particularly advantageous. Not only can recombinant antibodies be used wherever you would normally use a traditional monoclonal antibody, they also provide enhanced benefits in the field of therapeutics, being able to fuse with drugs and toxins. Many ScFvs are currently being used in clinical trials as a method to diagnose and treat illnesses - Currently over 30 are in clinical development. In addition, Fab recombinant antibodies have been used in a variety of different biosensors, including optical and electrochemical. Bispecific recombinant antibodies have also proved useful in the immunodiagnosis and immunotherapy of cancer and other diseases. Through genetic engineering, it is even possible to remove or add on key protein domains, allowing researchers to design antibody molecules with a variety of desired functions within one antibody.
Disadvantages of recombinant antibody production
However, there are also several disadvantages to recombinant antibodies and their production process:
Significant investment required
The technique required to create de novo recombinant antibodies takes time to learn. This can be a significant investment of resource, time and energy, particularly for smaller scale laboratories. In addition, in order to successfully create recombinant antibodies, an antibody gene library needs to be created. This enables researchers to then pick out the antibodies that bind to their target of interest. This takes a great deal of time and effort. Although this process can be bypassed by acquiring aliquots of a proven, pre-made library, this involves a significant cost, that many smaller laboratories may not be able to afford.
Expansive knowledge base needed
In addition to the time spent setting up and learning how to use an antibody gene library, a range of expertise is needed in techniques including bacterial transformation, phage amplification and DNA mutagenesis amongst others. These skills are necessary to select and screen antibodies from antibody libraries.
Labour intensive process
To increase the affinity and specificity of a monoclonal antibody is relatively simple - the host will create antibodies with ever greater affinity and specificity in response to repeated exposure to a particular antigen through the process of affinity maturation. However, to achieve recombinant antibodies with greater affinity and specificity, researchers have to carry out this process themselves, using advanced library construction and screening techniques, requiring a significant investment in time and resources to achieve.
A tool for the future?
There is a trend with recombinant antibodies being increasingly used as therapeutic and clinical tools. Many existing monoclonal antibodies have also been reverse-engineered into recombinant versions to improve their therapeutic properties. The ability of recombinant antibodies to be used as fragments and the ease in which these can be generated through the phase display methodology as well as their wide applications in the fields of cancer treatment and microbiology, suggests that the use of recombinant antibodies is only likely to grow over the next few years.