Which production method should be used?
Using mammalian systems such as rabbits, mice, hamsters etc. to produce antibodies, still dominates both initial antibody production and the ongoing expression within the biomanufacturing industry, as it is a tried and tested method. However, in recent years, new non-mammalian methods of both creating the initial antibody and the ongoing expression have been scaled up and are now available through commercial markets.
Knowing the advantages and disadvantages of each antibody production method is necessary to ensure you can make the right decisions in choosing which research tools to use in your experiments.
INITIAL MONOCLONAL PRODUCTION
Monoclonal antibody production: Mammalian systems still dominate
Using mammalian systems is the traditional method of monoclonal antibody production. These hybridoma cells lines, once created, can indefinitely produce antigen specific antibodies. It is a method known to produce effective antibodies with the appropriate post-translational modifications.
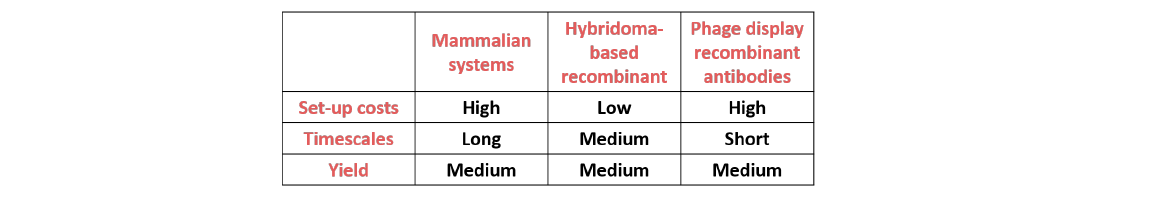
However, there are some disadvantages to this method. Production and selection of an effective hybridoma cell line can prove quite lengthy and expensive. Factors contributing to this include the use of animals and the high labour and production costs.
Recombinant methods: An alternative antibody production process?
Due to the negative aspects of using mammalian systems to produce monoclonal antibodies, some suppliers have turned to recombinant antibody technology. Recombinant antibodies can be generated by cloning the immune-specific heavy and light antibody chains into high yield expression vectors, which then allows expression of the antibody in a number of different systems including mammalian, bacteria or yeast hosts. Unlike the mammalian hybridoma cell lines, recombinant methods provide greater consistency and reproducibility across batches. In addition, the use of recombinant antibody technologies speeds up the antibody production process and can produce antibodies at a larger scale.
A key advantage of the recombinant antibody technology is the greater control it enables throughout the antibody production process, allowing environmental conditions to be amended to increase reproducibility. In addition, recombinant antibody production is relatively quick, with recombinant antibodies being produced in as little as eight weeks. The recombinant antibodies are also flexible and can easily be converted into different species, isotypes or subtypes by adding the appropriate domains. However, like other in vitro processes, a considerable amount of technical expertise is required as well as a large initial investment in time and money. This is offset by the fact that many antibodies can be produced rapidly and without the need for host animals once the process has been established.
Phage display methods: A change of pace
To overcome the challenges of labour intensive and time consuming antibody selection, Antibody Phage Display (APD) technology was developed. This method is based on cloning of amplified VH and VL fragment RT-PCR products into a phage display vector to produce a library of bacteriophage particles, each of which carries the genetic information for natural antibody sequences and displays the expressed monoclonal antibody on the phage surface. The bacteriophage library can be screened against an antigen of interest overnight. Once a specific bacteriophage has been shown to bind the antigen of interest the genetic information can be extracted and cloned into an expression vector as described above.
Table 1. Comparison of technologies for the selection and production of antibodies
Antibody expression
Once the hybridoma or recombinant antibody has been created, there are a variety of ways in which the antibody could be expressed.
Mammalian expression systems still dominate
Using mammalian expression systems is known to produce effective antibodies with the appropriate post-translational modifications which is important for modulating the binding and activity of an antibody. Transferring an antibody developed in a mammalian system to production in a prokaryotic system can result in changes in function and efficacy, due to the loss of mammalian post-translational modifications. Some mammalian post-translational modifications such as glycosylation can also improve antibody solubility and extend its half-life when used for in vivo applications. However, there are some disadvantages to this method. Factors contributing to this include the use of animals, limited hybridoma growth and antibody expression rates and the high production costs and difficulty in scaling up mammalian cell culture in comparison to prokaryotic or plant-based systems. Some researchers therefore choose instead to use alternative methods of antibody production that can provide antibodies within a shorter time scale and that may be considered more ethically friendly.
Microbial organisms: small but mighty?
Bacteria, yeast and fungi are being used in the production of transmembrane proteins and antibodies. In comparison to mammalian systems microbial organisms provide a higher yield, are easy to cultivate and can be scaled up easily at a low cost. There are however, a few difficulties in relying on microbial organisms for antibody expression as they are very susceptible to changes in the external environment and can be easily stressed. In addition, unlike mammalian systems, microbial organisms struggle to produce post-translationally modified antibodies and a rigorous, complex purification process is needed to process the antibodies.
Plants: an interesting new approach
Plants provide similar advantages to both mammalian and microbial methods of antibody production – they are abundant, grow quickly and have a similar endomembrane system and secretory pathway to human cells, so don’t trigger immune responses. In addition, like microbial organisms, plants provide a highly scalable, cost-effective and high yield method for antibody production. There are however, a number of regulatory uncertainties and questions around the suitability of plant glycans for human therapeutics.
Table 2. Comparison of systems for the production of antibodies

Conclusion - A choice of methods
There are a variety of different antibody production and expression methods that have been commercialised. In recent years, a greater diversification of antibody production methods has occurred. Every method though, has its own advantages and challenges that need to be considered to ensure your research tools are best suited for your experiments.
At Ximbio, we host a diverse range of antibodies that have been created and produced through a variety of different production methods. Discover antibodies produced through:
