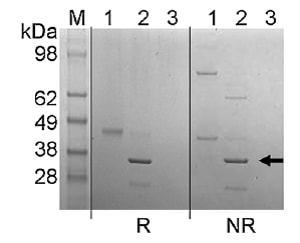
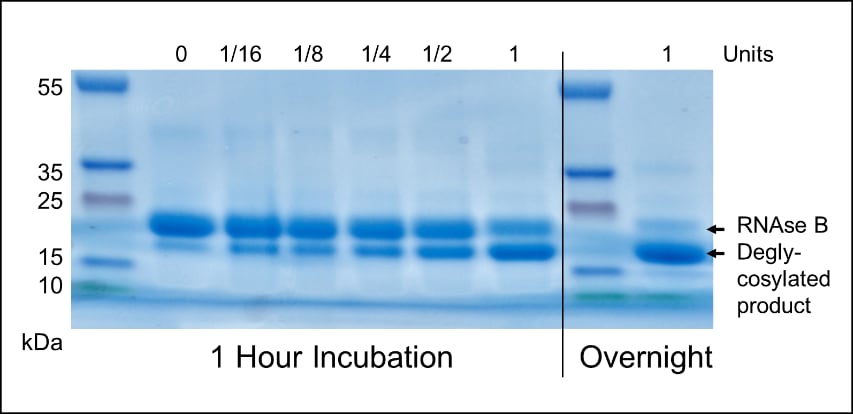
Endo-β-N-acetylglucosaminidase (Endo Tv), Enzyme
Invented by Dr Jared Gerlach from National University of Ireland, Galway
Invented at National University of Ireland, Galway
- Datasheet
- References (6)
- Inventor Info
Info
| Catalogue Number | 153542 |
| Source | Native enzyme isolated from Trichoderma viride protein mixture, derived from chitinase preparation. Purified by sequential anion exchange (AEC) and size exclusion (SEC) chromatography |
| Storage | 4oC (freezing not recommended) |
| Relevance |
This fungal endo-glycosidase (ENGase) from Trichoderma viride will release “Man5GlcNAc to Man9GlcNAc oligosaccharides from RNase B, ManGlcNAc, Man3GlcNAc, Man5GlcNAc, Man6GlcNAc and Man8GlcNAc from ovalbumin, and high-mannose and oligomannose structures from yeast invertase.”1 However, this enzyme will “not act on fucosylated, hybrid, complex-type or bisecting N-acetyl-glucosamine-containing N-linked oligosaccharide structures from ovalbumin, IgG and fetuin.”1 The exclusive preference of this enzyme “for high mannose type oligosaccharides without substitutions is in contrast to the bacterial ENGases, Endo H and Endo F1, which additionally cleave hybrid structures, and also tolerate fucosylation in the case of Endo H.”1 This enzyme has an ~Mr of 35 kDa as determined by SDS-PAGE. 1) Gerlach et al, Molecular BioSystems. 2012;8(5):1472-1481 • Cleaves within the chitobiose core of high- and oligomannose-type and N-linked oligosaccharides commonly found on a variety of glycoproteins • Can be used in preparative and analytical deglycosylation protocols and other applications involving high-mannose containing glycoproteins The use of Endo Tv in an analysis of the surface glycosylation of urinary extracellular vesicles is described in Gerlach JQ, Krüger A, Gallogly S, Hanley SA, Hogan MC, Ward CJ, Joshi L, Griffin MD. Surface glycosylation profiles of urine extracellular vesicles. PloS One. 2013 Sep 19;8(9):e74801. |
| Antigen/Gene or Protein Targets | Synonyms: β-N-Acetylglucosaminidase Tv • ENGase Tv • Glycopeptide-D-mannosyl-N4-(N-acetyl-D-glucosaminyl)2-asparagine 1,4-N-acetyl-beta-glucosaminohydrolase from T. viride • Putatively classified as EC 3.2.1.96 |
| Notes |
Applications Tested: • Comparative analysis of released distributions of high- and oligomannose type N-linked structures (Reference: Gerlach et al, Molecular BioSystems. 2012;8(5):1472-1481) • Modification of surface glycoproteins on intact extracellular vesicles (Reference: Gerlach et al, PloS One. 2013 Sep 19;8(9):e74801) Unit definition: One unit (U) will deglycosylate >90% of 10 µg native bovine ribo-nuclease B (RNase B) in 3 hours at pH 5.5 and 37 °C in a 15 µL reaction volume Unit assay protocol: •10 µL of 1 mg/mL native RNase B dissolved in PBS (10 mM sodium phosphate, 137 mM NaCl, 2 mM KCl, pH 7.4), 1.5 µL 10x reaction buffer (200 mM NaOAc or MES, pH 5.0), and 1.5 µL 10x broad spectrum protease inhibitor cocktail (Roche, EDTA free dissolved in water) were combined in a 300 µL thin-walled microcentrifuge tube. •To the above mixture, 2 µL neat Endo Tv solution (1 U) was added, mixed briefly by pipetting, sealed and placed in a 37 °C incubator for 3 h. •The enzymatic reaction was stopped by addition of 4 µL 5x SDS-PAGE loading buffer (25% β-mercaptoethanol, 50% glycerol, 5% SDS) and heating at 95 °C for 5 min. •The degree of deglycosylation was measured against untreated RNase B by mobility shift and densitometry following electrophoresis, Coomassie G250 staining and de-staining with 20% ethanol. Notes: •Denaturation of the substrate protein may reduce the time and amount of enzyme required to cleave oligosaccharides from a target protein, however it is recommended that denaturing agent be neutralized prior to adding the enzyme to prevent loss of activity. •Protease contamination has not been detected in 1 hour reactions containing RNase B. However, for extended incubation and in situations where recovery of intact protein are paramount, the addition of protease inhibitor is strongly recommended. Research Areas: • Direct glycoanalysis (e.g. release of carbohydrates followed by HPLC or MS) • Indirect glycoanalysis (e.g. modification of glycoproteins for MS/MS or affinity- based analysis methods such as lectin or antibody staining) Substrates: N,N'-diacetylchitobiosyl unit of non-fucosylated, high-mannose N-linked glycans attached to glycoproteins or glycopeptides Suggested dilution for release from soluble glycoprotein: 1U per 10 µg native glycoprotein, <15 µL reaction volume is optimal. |
| Positive Control | Bovine ribonuclease B (RNase B) |
| Research Area | Metabolism |
References: 6 entries
de la Torre-Escudero et al. 2019. PLoS Negl Trop Dis. 13(1):e0007087. PMID: 30657764.
Surface molecules of extracellular vesicles secreted by the helminth pathogen Fasciola hepatica direct their internalisation by host cells.
Europe PMC ID: 30657764
Surface glycosylation profiles of urine extracellular vesicles.
Europe PMC ID: 24069349
Gerlach et al. 2013. PLoS One. 8(9):e74801. PMID: 24069349.
Gerlach et al. 2012. Mol Biosyst. 8(5):1472-81. PMID: 22373601.
Differential release of high mannose structural isoforms by fungal and bacterial endo-β-N-acetylglucosaminidases.
Europe PMC ID: 22373601
Add a reference
References: 6 entries
de la Torre-Escudero et al. 2019. PLoS Negl Trop Dis. 13(1):e0007087. PMID: 30657764.
Surface molecules of extracellular vesicles secreted by the helminth pathogen Fasciola hepatica direct their internalisation by host cells.
Surface glycosylation profiles of urine extracellular vesicles.
Gerlach et al. 2013. PLoS One. 8(9):e74801. PMID: 24069349.
Gerlach et al. 2012. Mol Biosyst. 8(5):1472-81. PMID: 22373601.
Differential release of high mannose structural isoforms by fungal and bacterial endo-β-N-acetylglucosaminidases.
Add a reference