Cat. #154171
PARG Inhibitor PDD00017272 Small Molecule (Tool Compound)
Cat. #: 154171
Sub-type: Inhibitor
Availability: Please enquire for quantities and pricing
This fee is applicable only for non-profit organisations. If you are a for-profit organisation or a researcher working on commercially-sponsored academic research, you will need to contact our licensing team for a commercial use license.
Contributor
Inventor: Allan Jordan
Institute: Cancer Research UK, Manchester Institute
Tool Details
*FOR RESEARCH USE ONLY
- Tool name: PARG Inhibitor PDD00017272 Small Molecule (Tool Compound)
- Alternate name: Compound 34f, EP323273
- Research fields: Cancer;Genetics
- Tool sub type: Inhibitor
- Primary target: Poly(ADP-ribose) Glycohydrolase (PARG)
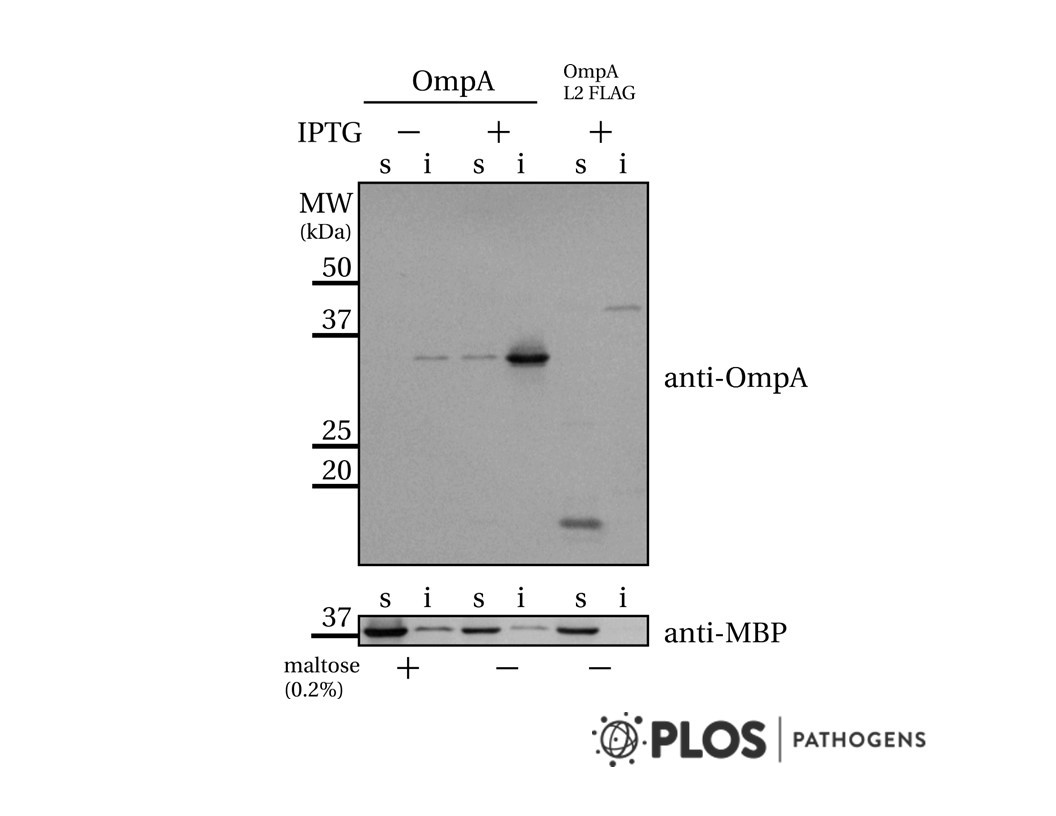
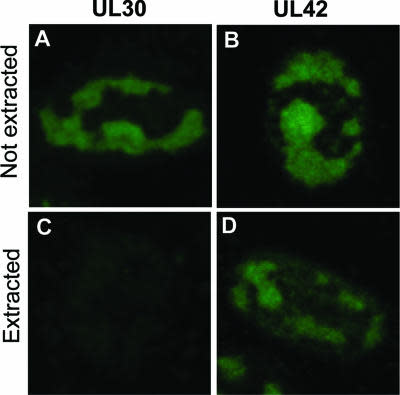
- Description: Exploiting the DNA damage response for cancer therapy relies on the fact that rapidly proliferating cancer cells have higher incidences of DNA damage, defective DNA repair pathways, genomic instability, or a combination of these facets. 1-3 Poly(ADP-ribosylation) is a post-translational modification that plays an important role in the repair of damaged sections of DNA.4-6 Poly(ADP-ribose) polymerases (PARPs), in particular PARP1, signal the presence of DNA damage and facilitate DNA repair. Upon DNA damage, PARP1 binds to single-strand break (SSB) sites and autoribosylates using NAD+ as a substrate to form poly(ADP-ribose) (PAR) chains. These PAR chains serve to recruit DNA repair proteins, such as XRCC1, to the site of DNA damage. Poly(ADP-ribose) glycohydrolase (PARG) systematically degrades the ADPribose polymers on the PARP enzyme, which is essential for DNA repair to occur; however, the precise order of events in this catalytic cycle is still unknown. Inhibiting PARG leads to the persistence of PAR chains, with predicted consequences on NAD recycling, PARP recycling, and SSB repair. Although PARP inhibitors have received much attention as cancer therapies, with olaparib approved by the FDA, the dearth of selective, cell-permeable small molecule PARG inhibitors has hampered assessment of the therapeutic potential of targeting PARG in human cancer. Additionally, while there are 17 known PARP family members, of which only a subset are inhibited by the PARP inhibitors, no close homologues of PARG exist. This provides an attractive target for drug discovery, given the cell's apparent reliance on this single enzyme in nuclear DNA damage repair.
- Purpose: Inhibitor
Handling
- Shipping conditions: Dry Ice
Target Details
- Primary target: Poly(ADP-ribose) Glycohydrolase (PARG)
References
- J. Med. Chem.2018612310767-10792