Cat. #151017
Anti-MBP [R29.6]
Cat. #: 151017
Sub-type: Primary antibody
Unit size: 100 ug
Availability: 3-5 days
Target: Maltose binding protein (MBP)
Class: Monoclonal
Application: ChIP ; IHC ; IF ; IP ; WB
Reactivity: Bovine
Host: Mouse
£300.00
This fee is applicable only for non-profit organisations. If you are a for-profit organisation or a researcher working on commercially-sponsored academic research, you will need to contact our licensing team for a commercial use license.
Contributor
Inventor: Julian Gannon
Institute: Cancer Research UK, London Research Institute: Clare Hall Laboratories
Tool Details
*FOR RESEARCH USE ONLY
- Name: Anti-MBP [R29.6]
- Alternate name: CCNA1; Cyclin A1; Testicular Tissue Protein Li 34; CT146
- Clone: R29.6
- Tool sub type: Primary antibody
- Class: Monoclonal
- Conjugation: Unconjugated
- Molecular weight: 53 kDa
- Reactivity: Bovine
- Host: Mouse
- Application: ChIP ; IHC ; IF ; IP ; WB
- Description: R29.6 is useful for detection and isolation of recombinant MBP fusion proteins.
- Immunogen: MOS maltose binding protein fusion protein
- Isotype: IgG1
- Myeloma used: Sp2/0-Ag14
- Recommended controls: MBP fusion protein generated with the pmal plasmid (New England Biolabs) in bacterial lysate.
Target Details
- Target: Maltose binding protein (MBP)
- Molecular weight: 53 kDa
- Tissue cell line specificity: MBP fusion protein generated with the pmal plasmid (New England Biolabs) in bacterial lysate.
- Target background: MBP is a bacterial protein commonly used as a fusion protein.
Applications
- Application: ChIP ; IHC ; IF ; IP ; WB
Handling
- Format: Liquid
- Concentration: 1 mg/ml
- Unit size: 100 ug
- Storage buffer: PBS with 0.02% azide
- Storage conditions: -15° C to -25° C
- Shipping conditions: Shipping at 4° C
References
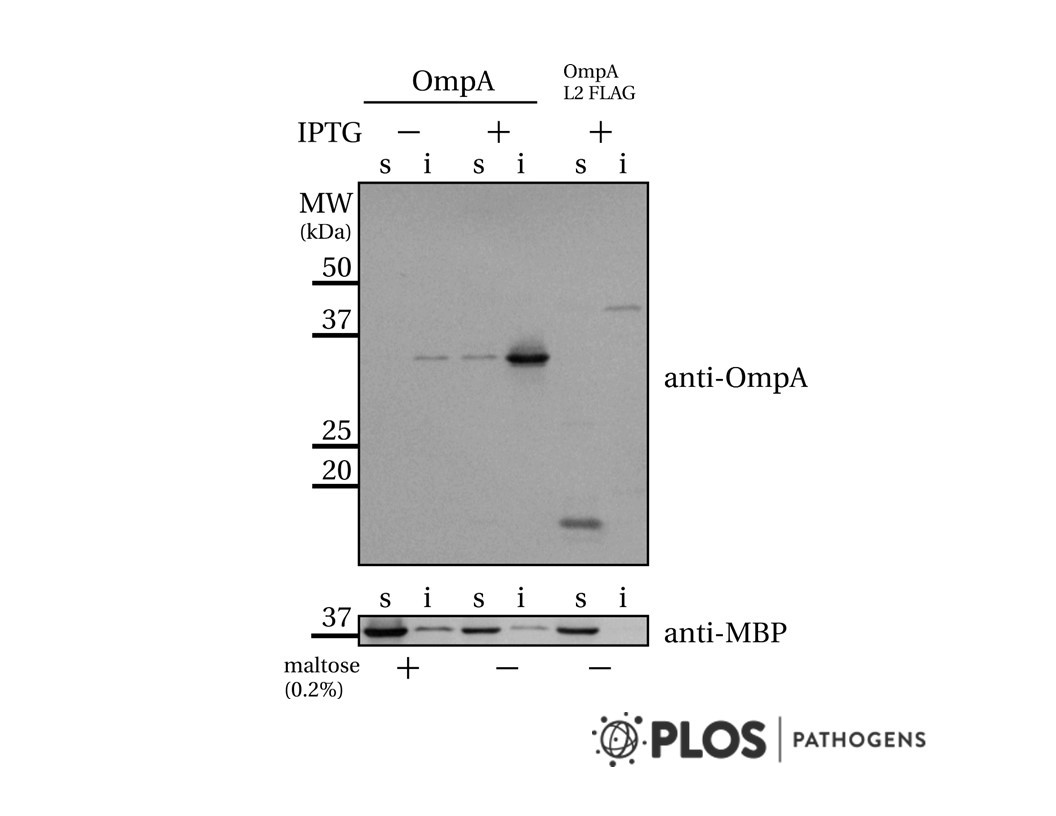
- Verhoeven et al. 2009. PLoS One. 4(8):e6739. PMID: 19707582.
- Differential bacterial surface display of peptides by the transmembrane domain of OmpA.
- Im et al. 2009. Dev Cell. 17(2):234-43. PMID: 19686684.
- Structure and function of the ESCRT-II-III interface in multivesicular body biogenesis.
- Liu et al. 2006. Genome Res. 16(12):1517-28. PMID: 17053089.
- Whole-genome comparison of Leu3 binding in vitro and in vivo reveals the importance of nucleosome occupancy in target site selection.
- Liu et al. 2005. Genome Res. 15(3):421-7. PMID: 15710749.
- DIP-chip: rapid and accurate determination of DNA-binding specificity.